Former Group Members
Post-doctoral researchers
Dr Xiaoyu Han
Xiaoyu worked on the surface chemistry of ThO2 and PuO2, especially their interactions with small molecules such as water and NOx. Her work formed part of the Nuclear Materials theme of the TRANSCEND consortium, and included two papers in the Journal of Nuclear Materials (Journal of Nuclear Materials 559 (2022) 153476 and Journal of Nuclear Materials 575 (2023) 154240). She left the group in February 2023.
Dr Victoria Berryman
Vikki ’s research interests involve using quantum mechanical-based methods, mainly density functional theory, to study the molecular structure and properties of metal-containing systems. She explored and characterized bonding in lanthanide and actinide complexes. The nature of these bonds is studied by exploring the effects of compressing metal-ligand bonds in molecules containing 4f- and 5f-block elements. Specifically, the presence of covalency is of interest in these systems. This project is part of the FORTRESS (F-Block Covalency and Reactivity Defined by Structural Compressibility) project, where the effect of high pressure in isostructural 4f- and 5f block molecules is being investigated, in collaboration with Profs Polly Arnold and Simon Parsons at the University of Edinburgh. Motivation for this project is the desire to separate long-lived minor actinides from spent nuclear fuel.
Vikki had previous experience using computational methods to investigate iron porphyrin systems, including exploration of spin state energetics; oxygen and nitric oxide bonding; and the effects of exchange mixing in hybrid density functional theory. She also studied other metalloporphyrin systems and the aqueous solvation of inorganic compounds.
Vikki left the group in August 2020.
Dr Adam Thetford
Adam’s research involved the interaction of actinides with minerals, which is key to understanding the environmental impact of An release. Adsorption of actinides on iron oxides surfaces are shown to co-ordinate differently depending on the actinide element (U, Np and Pu). The minerals being investigated are hematite and magnetite, with actinides in the form of uranyl and its Np and Pu analogues.
EXAFS and XANES can be used experimentally to find structure and oxidation state of these actinides on the mineral surfaces. The structures found via calculations are compared to experimental results by use of simulated EXAFS spectra.
Adam’s previous work has included the modelling to transition metal and metal oxide surfaces for catalysis on the surfaces focusing on supported Au catalysts.
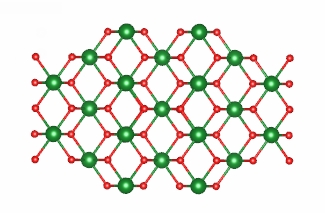
Hematite 0001 surface.
Adam left the group in May 2019.
Dr Bengt Tegner
More than 100 tonnes of separated plutonium are stored at Sellafield in the UK as PuO2 powder in sealed steel cans. Gas generation has occured in some of cans, with consequent can pressurization. Many routes to gas production have been suggested, several of which involve complex, inter-connected and poorly understood PuO2/H2O interactions.
PuO2 storage canisters.
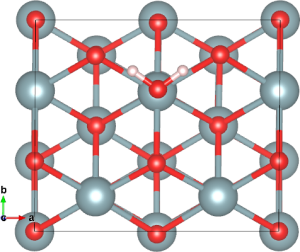
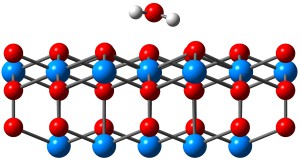
In work funded by the EPSRC’s DISTINCTIVE Consortium, and with further support from the Materials Chemistry Consortium, Bengt used plane wave DFT+U with periodic boundary conditions to study the adsorption of water on actinide dioxide surfaces, with the goal of understanding the processes involved in gas production. The optimised geometry of the {111} surface of UO2 with low water coverage is shown below:
Water adsorbed on the UO2 {111} surface.
Bengt joined the research group of Prof Stuart Macgregor in January 2018.
Dr Han-Shi Hu
Understanding chemical bonding is a central theme in chemical science. Due to complicated electron correlation effects and strong relativistic effects (including spin-orbit coupling), the bonding and electronic structures of heavy-element compounds are often difficult to measure, calculate and interpret. Advanced relativistic quantum chemistry approaches are needed to predict the properties of heavy-element systems.
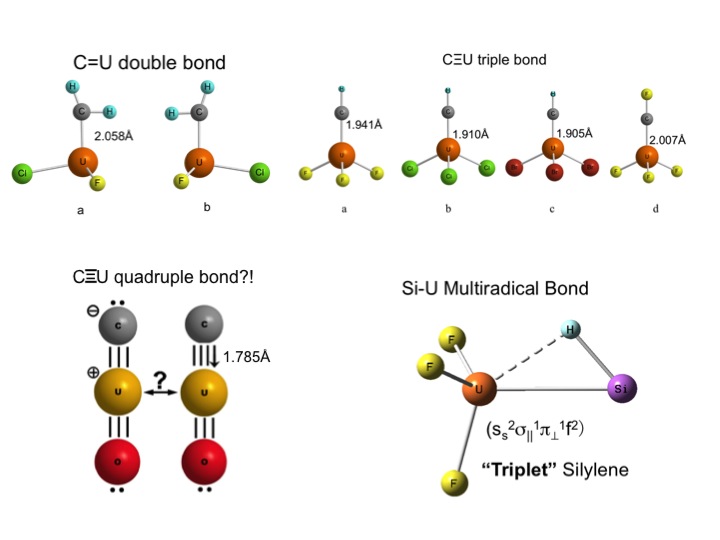
Prior to joining the group, Hanshi investigated the chemical bonding in heavy-element compounds via DFT and WFT methods, especially the multiple and multi-radical uranium bonds shown below [1, 2, 3, 4]. While in Manchester, she focused on the theoretical investigation of the structures, spectroscopy and reactions of other actinide compounds, especially those containing Th-Th bonds. She is also studying environmentally relevant uranyl peroxide compounds and nanoparticles using a grant of computing time at the CASCADE facility at the Environmental Molecular Sciences Laboratory in Washington State.
Han-Shi took up a faculty position at Tsinghua University in Beijing in January 2018.
Dr Daniel Reta (ORCID: 0000-0003-0000-9892)
Daniel’s research interests centre on the theoretical and computational study of the electronic structure of highly correlated systems. Before joining us, he dealt with the problem of accurately describing magnetic interactions in 3d coordination compounds and with the design of purely organic polyradicals with interesting magnetic properties (namely high-spin ground state and large ferromagnetic exchange coupling constants). Whilst in the group, Dani worked on uranium-based molecular systems, with the goal of establishing the most reliable computational approach to properly describe their structural, optical and magnetic properties.
Dani joined Dr Nick Chilton‘s research group in March 2017.
Dr Laura Estevez
Laura’s research interests centre on the computational study of the electronic structure of organometallic complexes. Before coming to Manchester, she focussed on understanding the chemical reactivity of transition metal complexes and the analysis of the Metal-Ligand chemical bonding by means of the QTAIM (The Quantum Theory of Atoms in Molecules) and NBO (Natural Bond Orbitals) procedures. Her project in our group focused on the theoretical investigation of the effect of high pressures on the compressibility of metal-ligand bonds in isostructural 4f- and 5f-block molecules within the FORTRESS (F-Block Covalency and Reactivity Defined by Structural Compressibility) project, in collaboration with Profs Polly Arnold and Simon Parsons at the University of Edinburgh.
Laura took up a position in Spain in September 2016.
Other former post-doctoral group members are:
Dr Andrew Kerridge, Dr Germán Cavigliasso and Dr Giuseppina Menconi
PhD students
Emma Pye

Radicals are highly reactive and ideal for building complex compounds. Samarium(II) diiodide, SmI2, also known as Kagan’s reagent, is a classic single electron transfer (SET) reagent that can efficiently produce radical species and mediate chemical processes that no other reagent can. It is a crucial catalyst in organic synthesis and one of the most significant SET reagents. However, a well-known drawback of SmI2, i.e., it must always be used in stoichiometric excess, raises issues of cost and waste and impedes its application in industry. Recently, the group of Prof. David J. Procter developed SmI2-catalysed reactions through radical relays that only need a catalytic amount of reagent. In these reactions, SET from SmI2 converts the substrate to a radical, which then undergoes radical relocation, bond-formation, new radical generation, and radical back-donation to regenerate the catalyst. Emma used density functional theory calculations and quantum chemical analyses to better understand the mechanisms driving these catalytic coupling reactions and radical relays.
Lin Yang
The search for molecules with ever increasing coordination number (CN) remains a very active area of research. In 2016, the record stood at 16 (in systems characterised both experimentally and computationally) but very recent computational predictions have raised the bar first to 17, and then 18, in complexes of helium with early actinide (An) cations. Furthermore, it has previously been shown that the electronic ground state of early actinide triatomic molecules can be altered by complexation with Noble gas (Ng) atoms. Lin employed a wide range of computational methods (including those based on DFT, coupled cluster theory, and PIMD) to search for stable An-Ng complexes with high CNs.
Following this work, Lin studied the migration of inert gas atoms through uranium nitride and plutonium-uranium mixed nitrides using periodic DFT.
Sophie Cooper
When it comes to f-block chemistry, the lanthanide series has been studied more fully than the actinides, and in particular, our knowledge of the role of the 5f electrons in bonding is incomplete. Moreover, the research that has been done on the 5f series has focussed on the earlier actinides such as uranium. Until recently it was believed that, unlike the earlier actinides, which appear to act somewhat like the transition metals, the later actinides behave similarly to the lanthanides. However recent studies have suggested that in fact these elements have a larger degree of covalency than the 4f elements and so are different to both the transition metals and lanthanides.
Sophie investigated the electronic structure and bonding of the later actinide elements through the use of DFT methods, including the role of 2+ oxidation states and covalency. Her work extended our knowledge of covalent bonding in the f block, and explored the fascinating chemistry of these largely unstudied elements. Her work on AnCl2 has been published, as has that on AnCl3.
Jon Tanti
Solutions to the long term storage of highly radioactive waste products, formed through the nuclear cycle, are required to allow for the continued safe use of nuclear energy. The long-term solution for the storage of highly radioactive waste must be stable and be able to withstand radiation damage, without destructive changes occurring in structural composition. Various forms of solid storage devices have been considered – such as glasses and ceramics which allow for the immobilisation of radioactive materials.
The ceramic zirconolite (CaZrTi2O7) has been the focus of experimental research for several decades and its candidacy as a potential nuclear waste-form has developed over time. Naturally occurring zirconolite has been found to contain actinides, such as uranium and plutonium, substituted onto the cationic sites. Using DFT within the Periodic Electrostatic Embedded Cluster Method (PEECM), Jon’s project focused on the effects of substitution of actinides into zirconolite.
Zirconolite.
Jon successfully defended his thesis in November 2021.
Naomi Ofili
Understanding the interaction between actinide elements and mineral surfaces is key to determining the fate of radionuclides should they escape into the environment. This knowledge can be used to develop strategies to prevent and control their migration by, for example, increasing the accuracy of models or identifying materials that can be used for radionuclide containment. Naomi’s project, funded by the Science and Technology Facilities Council, used computational quantum chemistry (plane-wave density functional theory within the periodic boundary condition framework) to model, for example, the adsorption of actinides on mackinawite.
Crystal structure of mackinawite.
Naomi successfully defended her thesis in August 2021.
Angelika Christodoulidou
Angelika’s aim was to study the adsorption mechanism of actinides (U, Np, Pu) on and into environmentally important minerals (metal oxohydroxides, clay etc). It is known that actinides can incorporate into mineral phases, and adsorption of actinides on and into geological materials has been suggested as a strategy to prevent the migration of actinides from nuclear waste storage. Hence, understanding actinide/surface interactions at the atomic scale is important both from a fundamental perspective and for the development and design of deep geological waste disposal facilities.
Angelika studied the interactions of uranyl and neptunyl with goethite (α-FeOOH), one of the most common forms of iron oxo-hydroxides, found in soils and sediments, and also adsorptions on calcite. The approach applied was hybrid DFT within the Periodic Electrostatic Embedded Cluster Method (PEECM).
Crystal structure of goethite.
Angelika successfully defended her thesis in January 2021.
Ben Atkinson
Ben focused on molecular f-block chemistry. For calculations on molecules containing f-elements, relativistic effects must be explicitly included in calculations and the near-degeneracy of many valence atomic orbitals results in many closely-spaced electronic states. These complications necessitate the use of ab initio wave functional theory to obtain a detailed understanding of the electronic structure of f-element containing molecules.
The first focus of Ben’s project was on molecules of the general formula EAnF3, where E = N, P, As, Sb and Bi; and An = U, Np, and Pu. Previous calculations suggest that NUF3, NNpF3 and NPuF3 have triple An-N bonds, however other electronic states (corresponding to the unpairing of electrons in the triple bond) are computed to be very close in energy. These excited states may match better with the experimental vibrational spectroscopy of these molecules [Inorg. Chem. 48 (2009) 6594], and so the true electronic structure is not clear. DFT and ab initio calculations were employed to gain a deeper understanding of these systems. Subsequent work focused on uranium-nitrogen chemistry, including an unusual U(V)-N2 system.
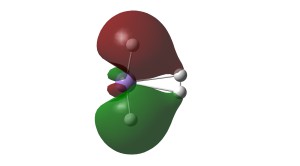
σ and π bonding Kohn-Sham MOs of N≡UF3.
Ben successfully defended his thesis in March 2021.
Jia-Li Chen
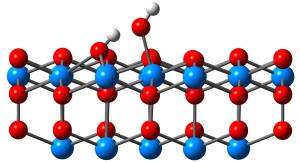
The actinide elements are important both fundamentally and for their special role in the nuclear fuel cycle. A common form of the actinide oxides in the solid state is as the fluorite AnO2, the properties of which attract much attention. The so-called minor actinides (neptunium, americium and curium) have been accumulated through the nuclear fuel cycle, and thus the study of these elements is important. Moreover, the interaction between water and the surface of minor actinide dioxides affects a variety of processes in the nuclear fuel cycle, especially for the storage of spent fuel. Jia-Li’s work focused on the theoretical study of minor actinide dioxide surfaces and the interaction of water on those surfaces.
The {111}, {110} and {100} surfaces of NpO2.
Jia-Li successfully defended her thesis in January 2021.
Jonathan Collard
Jonny’s project (part-funded by Sellafield Ltd.) employed hybrid DFT within the Periodic Electrostatic Embedded Cluster Method, and focused on the adsorption of water and HCl around oxygen vacancy defect sites in the {111}, {110} and {100} surfaces of PuO2. The overall purpose of the project is to find potential routes for the production of gas within the storage canisters mentioned above in Bengt’s section.
The {110} surface of UO2.
Jonny successfully defended his thesis in April 2020.
James Hales
Alternative fuels sources are an important area of research for finding efficient and cost effective ways to power our developing technology. Hydrogen fuel cells are a promising novel technology for powering a wide range of applications, but there are difficulties in using this approach for mobile systems; the aim of James’ project is to study computationally materials developed in the research group of Prof David Antonelli at the University of Lancaster, for safe and effective hydrogen storage. These materials exploit the Kubas interaction, and show much promise for improved hydrogen storage solutions. In work part-funded by our other collaborator on this project – Hydro-Quebec – James uses DFT to simulate molecular models of potential binding sites for dihydrogen in these transition-metal based materials.
Canonical Kubas binding orbitals for MnH2 binding to molecular dihydrogen. σ donation and π back donation. Hydrogen atoms in white and manganese in light purple.
James successfully defended his thesis in December 2019.
Sophie Sutherland-Harper
Sophie’s work (funded through the Next Generation Nuclear CDT by the EPSRC and Sellafield Ltd. and associated with the DISTINCTIVE consortium) is investigating surface interactions of Cl– and H2O with PuO2, as >5 tonnes of PuO2 has been stored in PVC bags since the 1970s and has become contaminated with Cl- and O-containing species. Heat treatment is the proposed method for removal of these species. The project involves experimentation on the metal oxide and contaminants at the National Nuclear Laboratory and the Institute for Transuranium Elements in Germany. CeO2, ThO2 and UO2 will also be studied as PuO2 analogues. The main analytical technique used is X-Ray Photoelectron Spectroscopy.
Degraded PVC bags used for storing PuO2 (Los Alamos).
Sophie successfully defended her thesis in December 2018.
Dr Kieran O’Brien
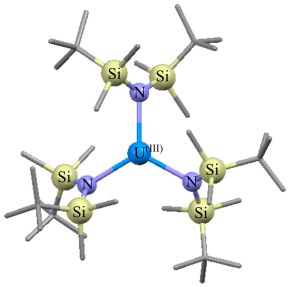
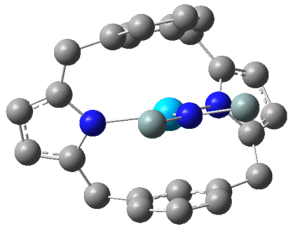
Kieran’s work focused primarily on hybrid DFT studies of a range of organoactinide complexes featuring the trans-calix[2]benzene[2]pyrrolide ligand (L2-), synthesised and ligated to thorium and uranium by Prof Polly Arnold’s group at the University of Edinburgh. The focus was on complexes of general formula LAnX, where the X ligand varies across the first row of the periodic table from boron-donor to oxygen-donor. Th(III), Th(IV) and U(III) were studied, and the L ligand was calculated in the η6κ1η6κ1 bonding mode, see below:
Schematic representation of the LAnX targets (a), and ball and stick image of the optimised geometry of LThN(SiH3)2 (b). Hydrogen atoms not shown.
Kieran’s targets provided an excellent opportunity to probe the electronic structure and bonding between the actinide and the L and X ligands. The principal tools employed in his study were the QTAIM and natural bond orbital approaches.
Kieran successfully defended his thesis in February 2018.
Dr Joseph Wellington
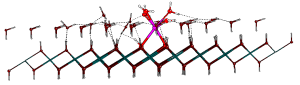
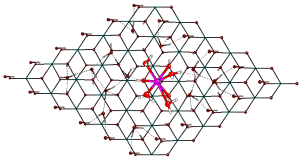
Joe’s project, part-funded by the National Nuclear Laboratory, was a study of the interactions of water with actinide dioxide surfaces, using the periodic electrostatic embedded cluster method (PEECM). This approach involves treating a small part of the surface as a molecular cluster using a quantum mechanical method (in this case hybrid DFT), whilst the rest of the system is approximated by point charges to reproduce the electrostatic interactions of the extended system with the cluster.
Embedding of quantum mechanical (QM) AnO2 cluster within a PEECM scheme. Left/top, QM cluster, actinide ions in blue, oxygen ions in red. Middle, QM cluster embedded in intermediate region of effective core (softening) potentials (black) and -2 point charges (small red), representing actinide and oxygen ions respectively. Bottom/right, QM cluster and intermediate region embedded in infinite array of +4 (small blue) and -2 (small red) point charges representing the actinide and oxygen ions respectively.
Joe investigated water adsorption on both the {111} and {110} surfaces of UO2 and PuO2, looking at the effects of surface coverage, surface termination and whether molecular or dissociative adsorption is more favourable. Some of his work was published in the Journal of Nuclear Materials.
Water molecule adsorbing molecularly (a) or dissociatively (b) on a U19O38 {111} surface cluster. Hydrogen atoms shown in white, oxygen in red and uranium in blue.
Joe successfully defended his thesis in July 2017.
Dr Eszter Makkos
Eszter’s work focused on gaining an improved understanding of the interactions between the principal fission products of uranium and the corroded surfaces of Magnox type fuel elements, and in part funded by the National Nuclear Laboratory and the Nuclear Decommissioning Authority.
Magnox (an Mg-Al based alloy) was used as a nuclear fuel cladding material in the first generation of British nuclear reactors. The spent uranium fuel rods and associated cladding are held in storage ponds at reactor sites and reprocessing facilities prior to reprocessing or final disposal. The corrosion of the cladding and resultant brucite (Mg(OH)2) sludge formation is a significant problem. Besides the corrosion products, the sludge contains leached actinides (238U, 239Pu and 241Am) and their fission products (mostly 137Cs and 90Sr) in significant concentrations which can form aquo and hydroxide complexes. Brucite has a hydroxide-terminated (0001) surface which is highly reactive, and it has been shown experimentally that it can absorb some of the above mentioned ions.
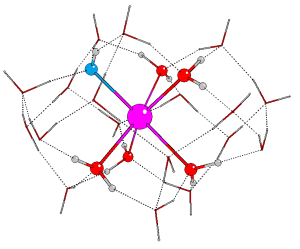
In first part of her project, Eszter used DFT to gain a better understanding of the microsolvation of Sr2+. Building on the previous work of Dr Andy Kerridge, she developed a model which combines implicit and explicit solvation to identify possible candidate species for surface adsorption.
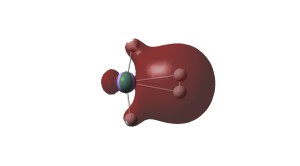
Ball and stick images of the optimised geometry of [Sr(OH)]+ with two explicit solvation shells, viewed from the side (a) and the top (b). The second shell waters are represented by tubes.
Eszter then moved on to investigate the adsorption of ions and their complexes on non-hydrated and hydrated Mg(OH)2 surfaces. Cluster-like surface models embedded in infinite point charges arrays were used (via the periodic electrostatic embedded cluster method (PEECM), as implemented in Turbomole 6.5) as well as periodic DFT simulations (in CRYSTAL14) to find the best representation of the surface and to study the interactions with the adsorbing ions. This work was also supported by the Materials Chemistry Consortium.
Ball and stick image of an optimised Sr2+ hydrate adsorbed onto a single layer of brucite covered with a monolayer of water; side view (a) and top view (b).
Eszter successfully defended her thesis in July 2017.
Dr Abigail Mountain
Abi’s research focused on the understanding of molecular organometallic systems and their reactions. In collaboration with the Mountford group at the University of Oxford she used a combined DFT, QTAIM and energy decomposition analysis approach to examine the electronic properties and reactivities of rare earth metal (Sc, Y, and Lu) boryl and gallyl complexes. The exploitation of unique properties of rare earth (RE) metals has allowed huge technological advances to be made in the last decade. However, the heavy use has led the US Department of Energy to develop a “Critical Materials Strategy” to safeguard the supply of RE metals. It is therefore imperative that we further our understanding of the physical and chemical properties of RE metal compounds in order to maximise the efficiency of their use.
Optimsed geometry of Sc{B(NArCH)2}{Me3SiCH2C(NCy)2}2(THF). Hydrogen atoms not shown.
Abi also conducted DFT studies of group 4 transition metal catalysed olefin polymerisation, in collaboration with LANXESS Elastomers B.V. who also funded her work. The project involved the polymerisation mechanism beginning from an activated charge-separated post-metallocene species; chain propagation, including the different region- and stereochemical possibilities; chain termination; and ion-pair interactions.
Abi also previously studied the correlations of QTAIM metrics with the strength of heavy element bonds.
Abi successfully defended her thesis in June 2017.
Dr Qian-Rui Huang
Spent nuclear fuels have attracted much interest due in part to the presence of the minor actinides (MAs, chiefly Am and Cm) which have high radioactive toxicity and very long lifetimes. Ideally we would wish to separate these from the many other elements present in the spent fuels (especially the lanthanide fission products). Several partitioning and transmutation strategies are being examined to effect this, one of which is to employ liquid extraction with ligands designed to selectively complex MAs in the presence of other cations. However, MA and lanthanide cations have very similar chemical properties in their dominant trivalent oxidation state in aqueous solution, leading to difficult chemical separation.
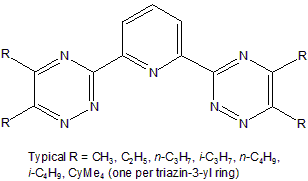
One possible appraoch is based on the SANEX (Selective ActiNide EXtraction) process, and work has shown that 2,6-bis(triazinyl)-pyridines (BTPs) and similar ligands have very good separation factors for the MAs from lanthanides, and are also both radiation and low pH tolerant.
2,6‑bis(5,6‑dialkyl‑1,2,4‑triazin‑3‑yl)pyridines.
The chemistry of the BTP family with trivalent f block cations has therefore been widely studied; nevertheless, the understanding of BTPs’ selectivity on a molecular level is still insufficient to confidently develop reagents or process conditions with higher efficiency. To provide enhanced understanding of the selectivity of BTP and related ligands for the MAs, Qian-Rui’s research focused on the nature of actinide-nitrogen bonding using DFT and the Quantum Theory of Atoms-in-Molecules (QTAIM). He found a good relationship between the QTAIM partial charges on the actinide atoms and calculated bond strengths. Moreover, he found strong correlation between the interaction energies of actinides to BTP-like polyazine ligands and to their single azine components.
Qian-Rui successfully defended his thesis in September 2016.
Other former PhD students are:
Dr Oliver Kirkby, Dr Claire Skipper, Dr Krishna Hassomal-Birjkumar, Dr Ian Kirker, Dr Matthew Tassell, Dr Chris Ryan, Dr Rosemary Coates, Dr Leif Jonasson, Dr Jonas Häller, Dr Luke Thomas, Dr Kieran Ingram, Dr Judy To, Dr Natalie Lambert, Dr Emma O’Flaherty, Dr Linnea Forslund, Dr Katherine Brown, Dr Maria Rosa Russo, Dr Carolyn Barker, Dr Ljuba Morris, Dr Sameer Bhambri and Dr Philip Champkin.