Chemical Breakthrough Award for Henry Moseley
Departmental Awards 28th September 2021
Henry Moseley’s 1913 paper on “The High-Frequency Spectra of the Elements” has been selected to receive one of 2020’s American Chemical Society’s Citation for Chemical Breakthrough awards.
The Department of Physics and Astronomy has received a Chemical Breakthrough Award 2020 from the American Chemical Society for the work of Henry Moseley.
The award program, from the Society’s Division of the History of Chemistry honours publications, patents and books that have made breakthroughs in chemistry and the molecular sciences that have been revolutionary in concept, broad in scope, and long-term in impact, and refers to Moseley’s publication, Philosophical Magazine Ser. 6 “The High-Frequency Spectra of the Elements,” 1913, 26, 1024-1034.
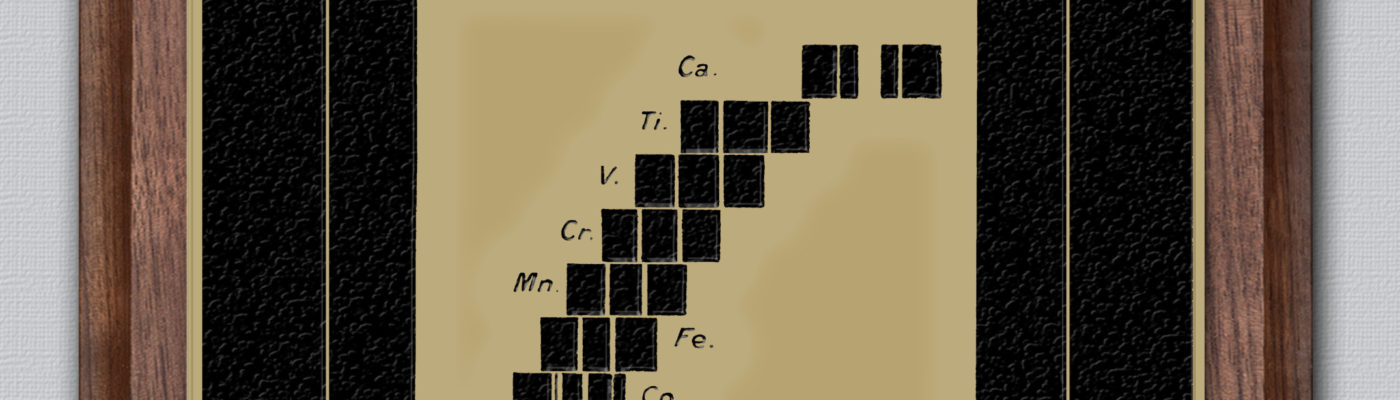
The paper details the observation and measurements of the X-ray spectra of various chemical elements found by the method of diffraction through crystals. At the time it was a pioneering use of the method of X-ray spectroscopy in physics. Moseley used Bragg’s diffraction law to determine the X-ray wavelengths, and through this discovered a systematic relationship between the wavelengths of the X-rays produced and the atomic numbers of the elements that were used as the targets in X-ray tubes.

This became known as Moseley’s law.
Before this discovery, the atomic number of an element had been thought to be a somewhat arbitrary sequential number, based on the sequence of atomic masses, occasionally modified where chemists found thought it appropriate. Particularly relevant is the work of Mendeleev, and his creation of the Periodic Table. Mendeleev had changed the orders of a few pairs of elements in order to put them in places he felt were more appropriate within the Table according to their chemical properties. Moseley’s experiments in X-ray spectroscopy indicated that some of these were correctly inferred, such as those for cobalt and nickel – confirming that they were in the right places in the Periodic Table (having the different atomic numbers, 27 and 28 respectively). His work also indicated that the Periodic Table of the time had gaps – which have been subsequently filled.
This paper and Moseley’s subsequent work demonstrated that the atomic numbers of elements are not arbitrary numbers based on chemistry and the intuition of chemists, but instead they have a firm experimental basis from the physics of their X-ray spectra.

Whilst Moseley’s life was tragically cut short in World War I, there is no doubting his impact on the work of generations following his research. And that this award is very gratefully received from the American Chemical Society on his behalf. The plaque can be found displayed in the Schuster Building foyer next to the Henry Moseley Lecture Theatre.
This is The University of Manchester’s fourth Citations for Chemical Breakthrough Award, with previous awards honouring E. Frankland (1852), E. Rutherford (1911) and M. G. Evans and M. Polanyi (1935).
ACS AwardsAmerican Chemical SocietyChemical Breakthrough AwardCitation AwardInternational Awards

Leave a Reply